ギャラリー(Gallery)
■ Our research was featured on the inside front cover of Small
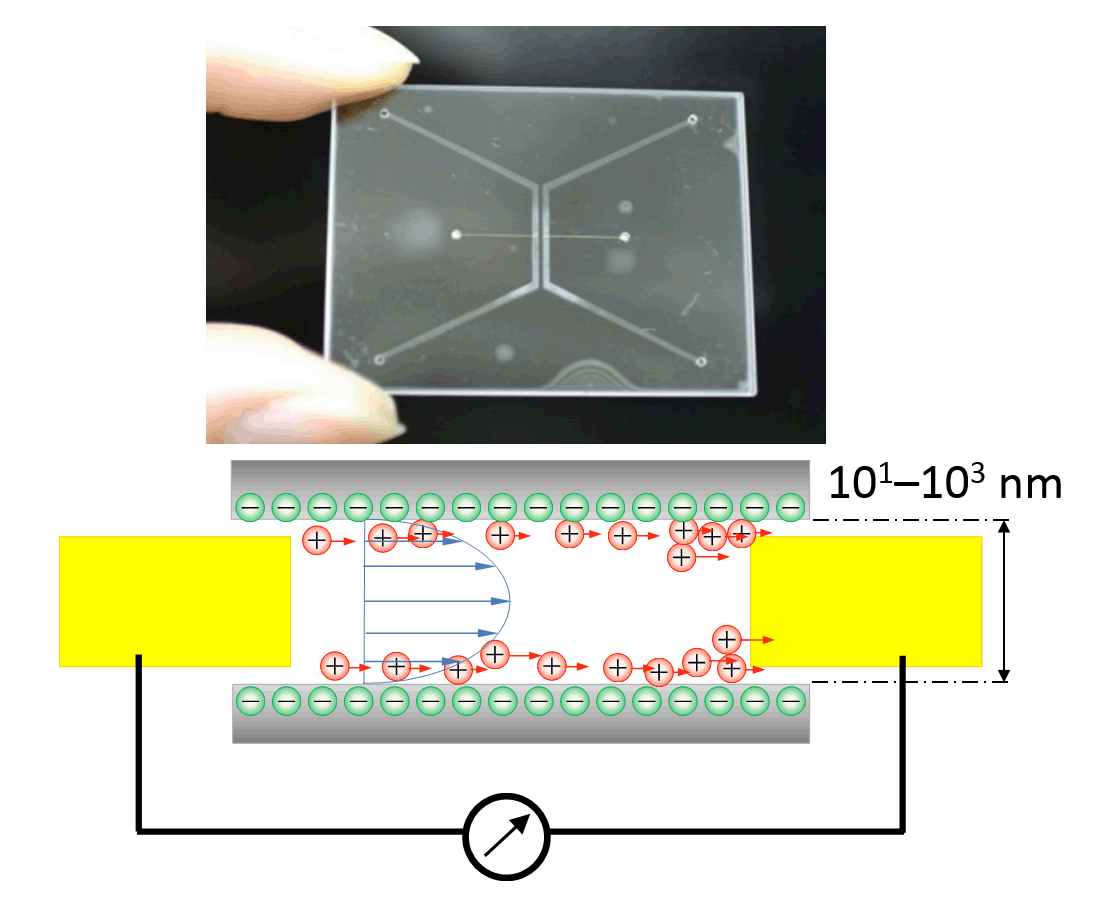
Using a pair of nanoelectrodes embedded within a tiny nanochannel space
on a novel nanofluidic chip, Y. Xu and B. Xu demonstrate the in-situ probing
of anomalous electrokinetic behaviors of water confined in nanospaces.
On page 6165, these measurements provide the first in-situ experimental
evidence for the existence of the unusual nature of water confined in nanospaces.
The understanding gained is very helpful for elucidating physical, chemical,
biological, and life phenomena occurring in small spaces.
Small, 2015, doi: 10.1002/smll.201570275
In-situ measurement of streaming currents in a single nanochannel with
femtoliter-scale volumes is achieved using an elaborately designed and
fabricated glass nanofluidic chip with probe electrodes embedded within
the nanochannel. The device and the method suggest a useful nanoscale tool
enabling in situ understanding of the unique liquid properties observed
in nanofluidic channels. Small, 2015, (doi:10.1002/smll.201502125)
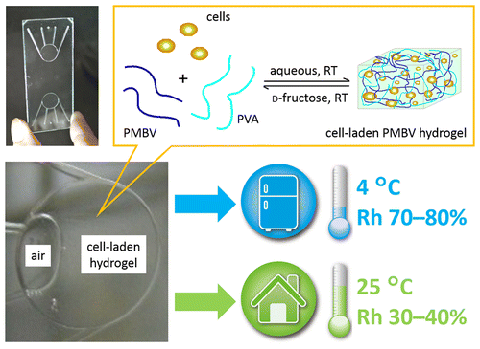
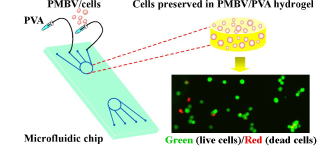
We report spontaneous packaging and hypothermic storage of mammalian cells
under refrigerated (4 °C) and ambient conditions (25 °C) using a cell-membrane-mimetic
methacryloyloxyethyl phosphorylcholine (MPC) polymer hydrogel incorporated
within a glass microchip.The approach would be a promising miniature and
portable solution for flexible supply and delivery of small amounts of
cells from bench to bedside. ACS Applied Materials & Interfaces, 2015, (doi:10.1021/acsami.5b06796)
■ Our research was featured on the inside back cover of Lab on a Chip
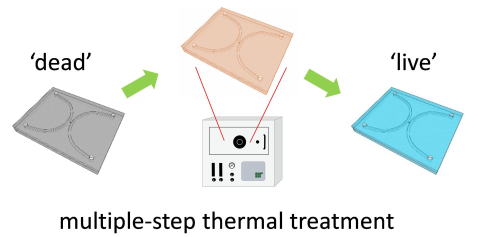
A simple method for the total regeneration of glass nanofluidic chips was
described. The method provides a technical solution to significantly improve
the reusability of glass nanofluidic chips and will be useful for the promotion
and acceleration of research and applications on nanofluidics.
Lab on a Chip, 2015, 15, 3856-3861 (doi:10.1039/C5LC00604J)
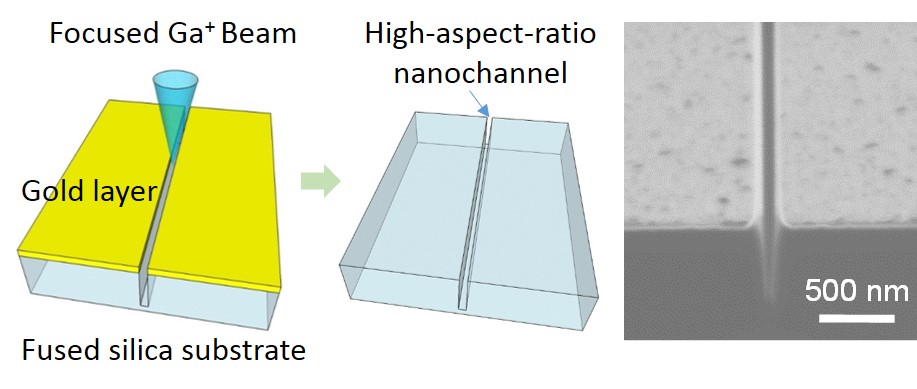
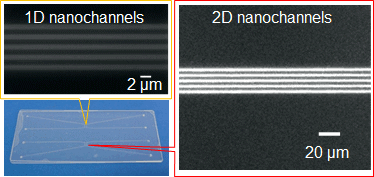
Nanochannels fabricated in fused silica substrates are ideal tools for single (bio)molecular studies in biology and are promising in the development of innovative applications in chemistry. To obtain a higher throughput and a higher level of integration and functionalization, nanochannels with high aspect ratios and nano-in-nano structures are very much desired, but their fabrication is a challenge. Herein, we report a method for fabricating nanochannels with such challenging critical dimensions and structures in fused silica substrates by using a focused ion beam.
RSC Advances, 2015, 5, 50638-50643. (doi: 10.1039/C5RA06306J)
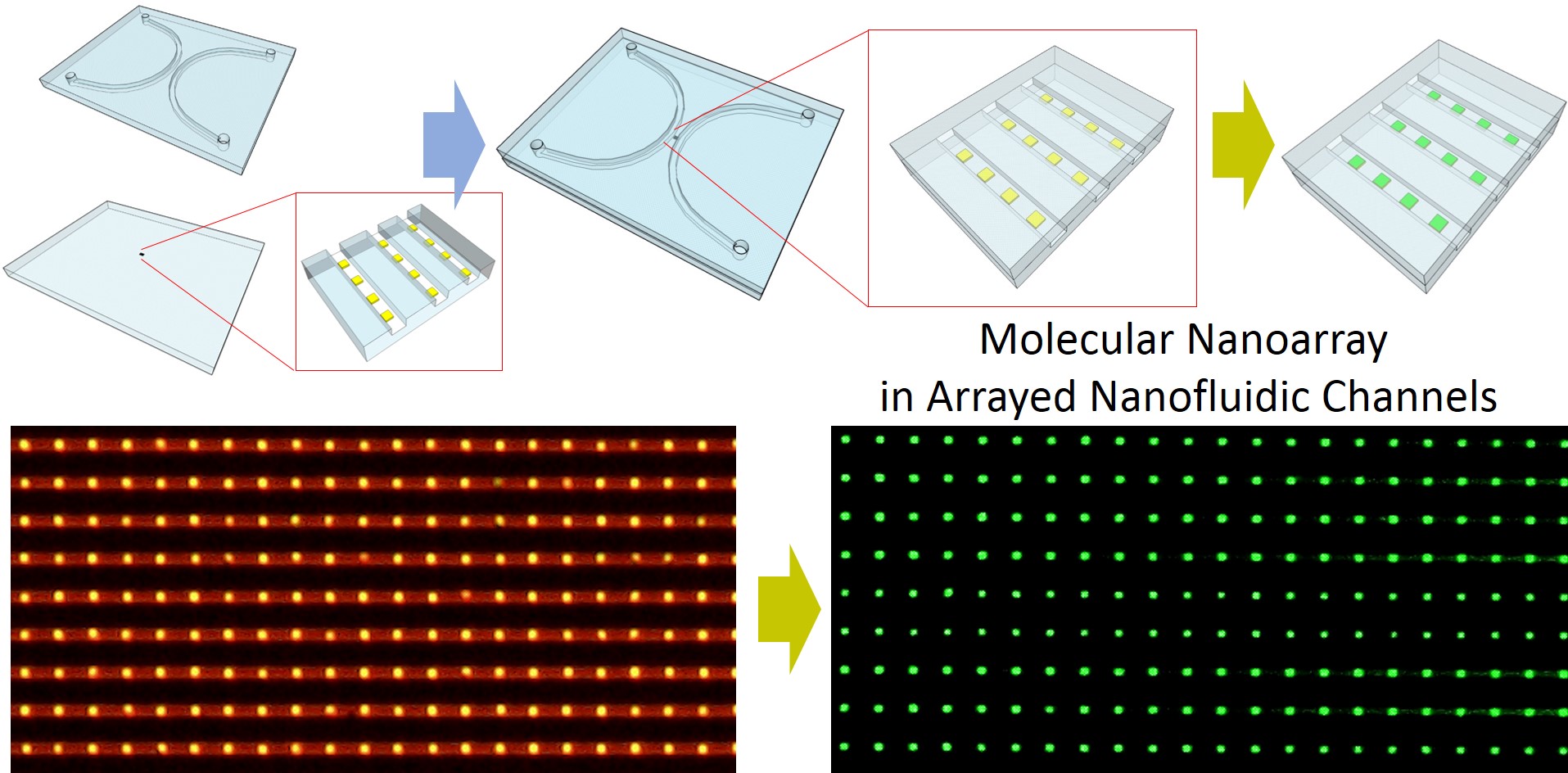
■ Our research was featured on the inside back cover of Lab on a Chip
A general method for site-specific nanopatterning of functional metallic
and molecular arbitrary features in glass nanofluidic channels was established.
The method opens the way for precise functionalization of nanofluidic channels,
which has been greatly challenging in the field of nanofluidics.
Lab on a Chip, 2015, 15, 1989-1993. (doi: 10.1039/C5LC00190K)
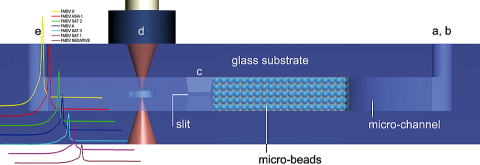
A microchip-based enzyme-linked immunosorbent assay (micro-ELISA) method
capable of detecting the cattle foot-and-mouth disease virus(FMDV) was
developed.
The strong, nanostructure-friendly, and high pressure-resistant bonding of glass nanofluidic chips at room temperature by an O2/CF4 gas mixture plasma treatment is achieved
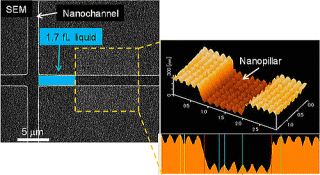
The basic principle of a Laplace nanovalve was verified, and a 1.7 fL
droplet (water in air) was successfully generated and handled for the first
time.
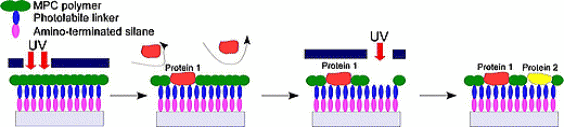
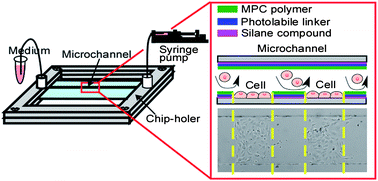
This technique was based on combining 2-methacryloyloxyethyl phosphorylcholine
(MPC) polymer, which is known to non-biofouling compound, photocleavable
linker (PL) that was modified to localize cells and to connect between
MPC polymer and amino-terminated silanized surface. Using ultraviolet (UV)
light illumination, MPC polymer can be selectively eliminated by photochemical
reaction in order which resulted in micropatterning of multiple biomolecules
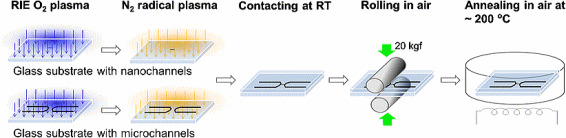
Direct bonding of fused silica glass nanofluidic chips at low temperature,
around 200 °C in ambient air, through a two-step plasma surface activation
process which consists of an O
2 RIE plasma treatment followed by a nitrogen MW radical activation
■ Our research was highlighted on the cover of Analytical Sciences.
We modified a newly designed microchip for single-cell analysis and
regulated the cell-adhesive area inside a cell-chamber of the microfluidic
system by using surface-modification techniques involving a silanization
compound, a photo-labile linker and the 2-methacryloyloxyethyl
phosphorylcholine (MPC) polymer covalently bonded on the surface of a
microchannel. Analytical Sciences, 2011, 27, 973-978. (doi:10.2116/analsci.27.973)
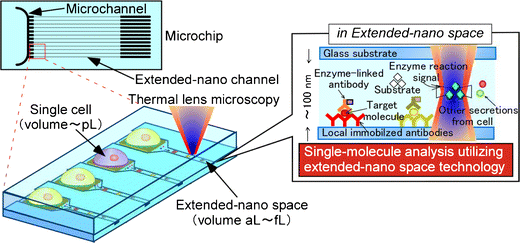
By combination of cell technology and microchip technology, innovative
cellular biochemical tools can be created from the microscale to the nanoscale,
for both practical applications and fundamental research. The image demonstrates
a concept of analysis of a single cell at the single molecule level on
a microchip with integration of extended-nano (101-103 nm) space.
In order to tackle both regional and global foot-and-mouth disease virus (FMDV) epdimics, we hereby develop a rapid microfluidic thermal lens microscopic method to screen swine type O FMDV with good efficiency. The scheme has great merits in terms of field portability, sample volume, assay time, analytical sensitivity, and test reproducibility.
■ Our research was highlighted on the cover of Advanced Materials.
We report on a phospholipid polymer hydrogel that exhibits a remarkable
ability to preserve cells on microfluidic chips without perfusion culture
under cell-based assay conditions, with characteristics including maintenance
of high cell viability, restraint of cell proliferation, and minimization
of cellular function loss over a period of days and weeks. This material
could establish a revolutionary flexibility for cellbased applications.
Advanced Materials, 2010, 22, 3017-3021. (DOI: 10.1002/adma.201000006)
A simple and direct approach for cell patterning inside the microchannel
using photochemical reaction.
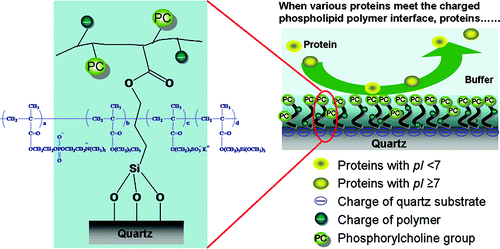
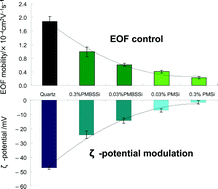
High capability of a charged interface to suppress adsorption of both anionic and cationic proteins was reported.
■ Our research was highlighted on the cover of Lab on a Chip
Charged phospholipid interfaces in microchannels allow the simultaneous
control of multiple electroosmotic flows and simultaneously suppress non-specific
bioadsorptions. Lab on a Chip, 2007, 7,199-206. (DOI: 10.1039/B616851P)